24
2024
-
02
Efficient mastery of carbon source and alkalinity calculation, elevating nitrification and denitrification to new heights!
Author:
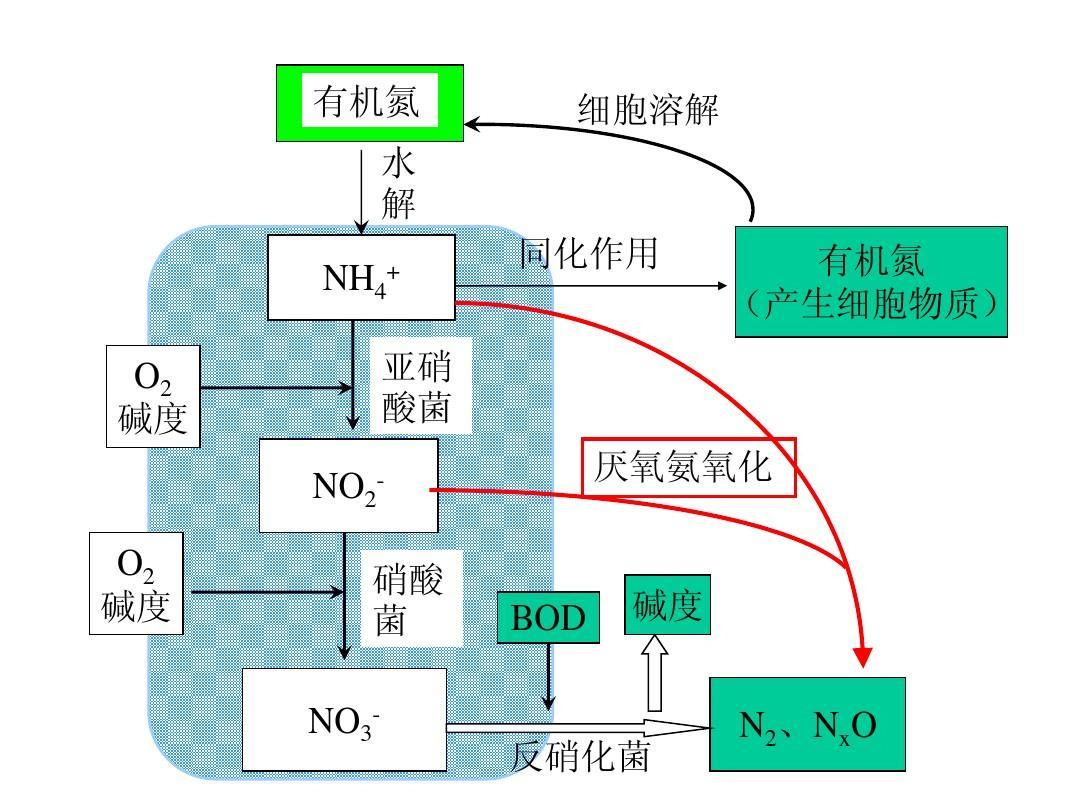
Nitrification reaction process of nitrifying bacteria: Under aerobic conditions, ammonia nitrogen is oxidized by nitrifying bacteria to form nitrite and nitrate. It includes two basic reaction steps: the reaction in which nitrite bacteria (Nitrosomonas sp) participate in the conversion of ammonia nitrogen to nitrite; Nitrobacter sp is involved in the reaction of converting nitrite into nitrate. Nitrobacillus and Nitrobacillus are chemoautotrophic bacteria that use CO2, CO32-, HCO3-, and other carbon sources to obtain energy through the redox reactions of NH3, NH4+, or NO2-. The nitrification reaction process needs to be carried out under aerobic or Oxic conditions, with oxygen as the electron acceptor and nitrogen as the electron donor. The corresponding reaction formula is: nitrite reaction equation: 55NH4++76O2+109HCO3 → C5H7O2N+54NO2-+57H2O+104H2CO3 Nitrification reaction equation: 400NO2-+195O2+NH4-+4H2CO3+HCO3- → C5H7O2N+400NO3-+3H2O Nitrification process total reaction formula: NH4-+1.83O2+1.98HCO3 → 0.021C5H7O2N+0.98NO3-+1.04H2O+1.884H2CO3 Through the material balance calculation of the above reaction process, it can be concluded that in the nitrification process, To oxidize 1 gram of ammonia nitrogen to nitrate nitrogen, aerobic 4.57 grams are required (including 3.43 grams of oxygen consumption for nitrite reaction and 1.14 grams of oxygen consumption for nitrification reaction), and approximately 7.14 grams of bicarbonate (calculated as CaCO3) are required for alkalinity. During the nitrification reaction, the transformation of nitrogen elements undergoes the following processes
Several processes: ammonia ion NH4->hydroxylamine NH2OH ->nitrosyl NOH ->nitrite NO2->nitrate NO3-.
Denitrifying bacteria denitrification reaction process: Under anaerobic conditions, denitrifying bacteria are used to reduce nitrite and nitrate to nitrogen and escape from anhydrous water, thereby achieving the goal of nitrogen removal. Denitrification is the process of reducing nitrate and nitrite produced during the nitrification reaction to nitrogen gas. Denitrifying bacteria are a type of chemotrophic heterotrophic and anoxic microorganisms. When there is molecular oxygen present, denitrifying bacteria oxidize and decompose organic matter, using molecular oxygen as the final electron acceptor. When there is no molecular oxygen present, denitrifying bacteria use N3+and N5+in nitrate and nitrite as electron acceptors, O2- as hydrogen acceptor to generate water and OH - alkalinity, and organic matter as carbon source to provide electron donor energy and achieve oxidation stability. Therefore, it can be concluded that denitrification reaction must be carried out under anaerobic conditions. The process of reducing NO3- to N2 is as follows: NO3- → NO2- → NO → N2O → N2 During denitrification, denitrifying bacteria require organic carbon sources (such as carbohydrates, alcohols, organic acids) as electron donors to use the oxygen in NO3- for anaerobic respiration. The reaction process can be simply represented by the following equation: NO3-+4H (electron donor organic compound) → 1/2N2+H2O+2OH-NO2-+3H (electron donor organic compound) → 1/2N2+H2O+OH - Carbon containing organic compound in wastewater as the electron donor in the denitrification reaction process. According to the above equation, for every 1g of NO2- to N2 conversion, 1.71g of organic matter (represented by BOD) is required; When converting 1g of NO3- to N2, 2.86g of organic matter (expressed as BOD) is required. Simultaneously producing 3.57g of bicarbonate alkalinity (with CaCO3). If dissolved oxygen is present in wastewater, the required carbon source organic matter (represented by BOD) for complete denitrification is calculated using the following formula: C=2.86Ni+1.71N0+DO0, where C is the amount of organic matter required for denitrification process (represented by BOD), mg/l; Ni is the initial nitrate nitrogen concentration (mg/l), N0 is the initial nitrite nitrogen concentration (mg/l), and DO0 is the initial dissolved oxygen concentration (mg/l). If the carbon source organic matter concentration in wastewater is insufficient, easily biodegradable carbon source organic matter (methanol, ethanol, or sugars) should be added. Taking methanol as an example, NO3-+1.08CH3OH+0.24H2CO3->0.056C5H7O2N+0.47N2 ↑+1.68H2O+HCO3- If there is NO2- in water, the following reaction will occur: NO2-+0.67CH3OH+0.53H2CO3->0.04C5H7O2N+0.48N2 ↑+1.23H2O+HCO3- As can be seen from the above formula, 1.53g and 2.47g of methanol are required for each reduction of 1g NO2- and 1g NO3-, respectively. When dissolved oxygen is present in water, the reaction formula for oxygen consumption of methanol is: O2+0.93CH3OH+0.056NO3- → 0.056C5H7O2N+1.64H2O+0.056HCO3-+0.59H2CO3
In summary, the formula for the dosage of organic carbon source (methanol) required in the denitrification process is: Cm=2.47Ni+1.53N0+DO0. Among them, Cm is the required methanol concentration (mg/l) in the denitrification process. Other symbols are the same as above. The nitrification reaction consumes 4.57g of oxygen and 7.14g of alkalinity per 1g of ammonia nitrogen oxidation, which is manifested by a decrease in pH value. During the denitrification process, nitrate nitrogen is removed while carbon source is removed, and this part of carbon source is equivalent to DO2.6g. In addition, Compensation alkalinity of 3.57g during denitrification process.
Related Products
Biological nitrogen removal process of low temperature wastewater
2024-05-28
Prevention and treatment of calcium carbonate scaling in reverse osmosis operation
2024-05-22
Treatment of pyrazolone production wastewater - bipolar membrane electrodialysis process
2024-05-20
How much salt does sewage contain that can enter the biochemical system?
2024-05-17
Huanke Environmental Protection Technology
HOTLINE:
Address:Gongye 1st Street, Weicheng District, Weifang City, Shandong Province China
Contact:Zhang Gong
Phone:+86-18865361829
Email:sdhuanke@163.com


Consult
Copyright © 2023 Shandong Huanke Environmental Protection Technology Co., Ltd