19
2024
-
01
Why is the total nitrogen in your sewage treatment not up to standard? Super instance interpretation!
Author:

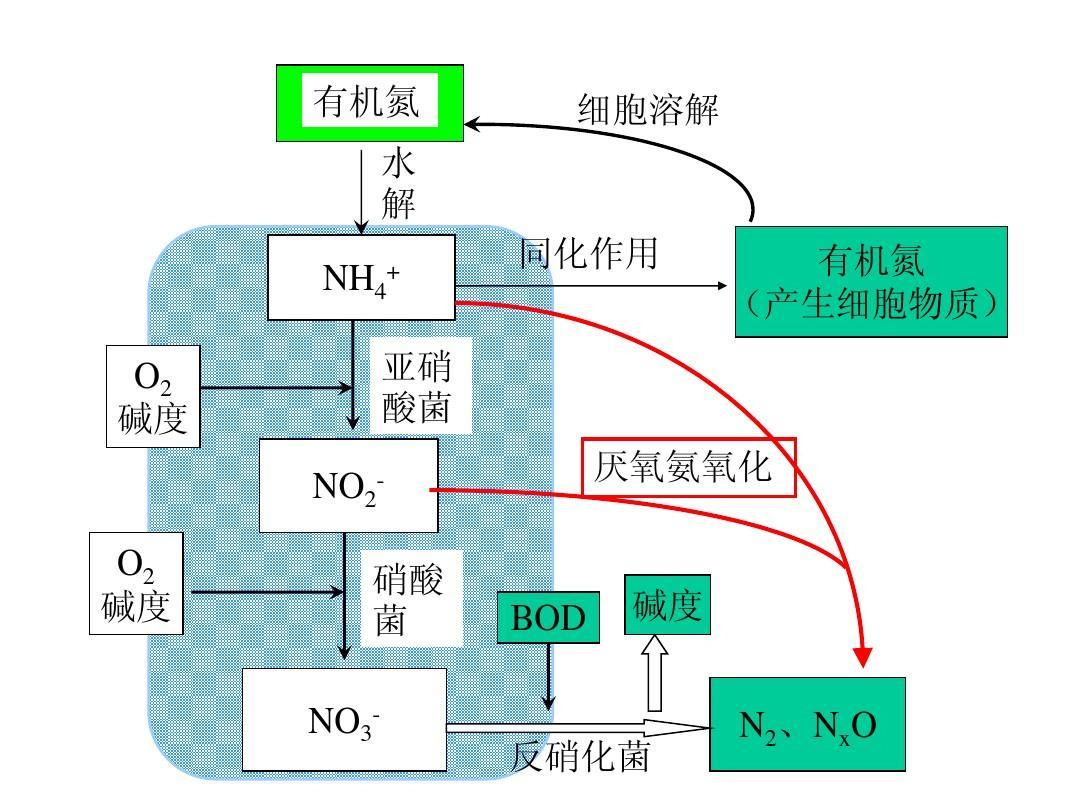
1. Excessive ammonia nitrogen caused by organic matter
For high ammonia nitrogen wastewater with a CN ratio less than 3, carbon sources need to be added to improve the completeness of denitrification because the denitrification process requires a CN ratio of 4-6. At that time, the carbon source added was methanol. For some reasons, the outlet valve of the methanol tank fell off, and a large amount of methanol entered the tank A, which led to a lot of foam in the aeration tank, the COD and ammonia nitrogen in the effluent soared, and the system collapsed.
Analysis: A large amount of carbon sources enter pool A, but denitrification cannot be utilized. When entering the aeration tank, due to sufficient substrate, heterotrophic bacteria have aerobic metabolism, consuming a large amount of oxygen and trace elements. Because nitrifying bacteria are autotrophic bacteria with poor metabolic ability, oxygen is contested and cannot form dominant bacterial species, so nitrification reaction is limited and ammonia nitrogen increases.
Solution:
1. Immediately stop water ingress for suffocation and continuous opening of internal and external backflow
2. Stop pressing sludge to ensure sludge concentration
3. If organic matter has caused non filamentous bacteria to swell, PAC can be added to increase sludge flocculability, and defoamer can be added to eliminate impact foam
2. Excessive ammonia nitrogen caused by internal reflux
There are two reasons for the ammonia nitrogen exceeding the standard caused by internal reflux that the author has encountered so far: electrical failure of the internal reflux pump (there is a running signal due to on-site tripping), mechanical failure (impeller detachment), and human factors (the internal reflux pump has not been tested for positive and negative rotation, and the site is in a reverse state).
Analysis: The excessive ammonia nitrogen caused by internal reflux can also be attributed to organic matter impact, because there is no reflux of nitration solution, resulting in only a small amount of nitrate nitrogen carried by external reflux in tank A. The overall environment is anaerobic, and the carbon source only hydrolyzes and acidifies without completely metabolizing into carbon dioxide and escaping. So a large amount of organic matter enters the aeration tank, leading to an increase in ammonia nitrogen.
Solution:
The problem of internal reflux is easy to detect, and it can be determined whether it is caused by internal reflux through data and trends: in the initial stage, the outlet nitrate nitrogen of tank O increased, the nitrate nitrogen of tank A decreased until 0, and the pH decreased. Therefore, there are three solutions:
1. Promptly identify the problem and inspect the internal reflux pump
2. The internal reflux has caused an increase in ammonia nitrogen. Repair the internal reflux pump, stop or reduce the inlet water for suffocation and explosion
3. The nitrification system has collapsed, and the water supply has stopped bursting. If conditions are urgent, similar biochemical sludge from the denitrification system can be added to accelerate system recovery.
3. Excessive ammonia nitrogen caused by low pH
There are three situations where the author currently encounters ammonia nitrogen exceeding the standard due to low pH:
1. If the internal reflux is too large or the aeration opening at the internal reflux is too large, it can carry a large amount of oxygen into pool A, damage the anaerobic environment, and cause aerobic metabolism of denitrifying bacteria. Some organic matter is metabolized by aerobic metabolism, seriously affecting the integrity of denitrification. Denitrification can compensate for the metabolism of half of alkalinity in nitrification, so the destruction of the anaerobic environment leads to a decrease in alkalinity and pH, The nitrification reaction is inhibited and ammonia nitrogen increases below the appropriate pH for nitrifying bacteria. Some colleagues may encounter this situation, but they have never found the reason in this regard.
2. Insufficient influent CN ratio is also due to incomplete denitrification, resulting in low alkalinity and a decrease in pH.
3. The continuous decrease in pH caused by the decrease in influent alkalinity.
Analysis: The probability of ammonia nitrogen exceeding the standard caused by a decrease in pH is relatively low in practice, as the continuous decrease in pH is a process. Generally, operators start adding alkali to regulate pH before finding the problem
Solution:
1. The problem of low pH is actually very simple, which is to start adding alkali to maintain pH when it continuously decreases, and then analyze to find the cause.
2. If the pH is too low and has already caused the system to collapse, the author has been exposed to situations where the nitrification system has not yet collapsed when the pH is between 5.8 and 6. However, in order to replenish the pH in a timely manner, the first step is to replenish the system's pH, and then suffocate or add the same type of sludge.
4. Excessive ammonia nitrogen caused by low DO
The wastewater that the author has operated on is high hardness wastewater, which is particularly prone to scaling. When using a microporous aerator for aeration, the aeration head will become blocked after running for a period of time, resulting in the inability to lift DO and an increase in ammonia nitrogen.
Analysis: The reason is simple. The function of aeration is to oxygenate and stir, and the blockage of the aeration head affects both. Nitrification reaction is aerobic metabolism, and it needs to be ensured that the dissolved oxygen in the aeration tank is suitable for normal operation. However, if the DO is too low, nitrification will be hindered and ammonia nitrogen will exceed the standard.
Solution:
1. Replace the aeration head. If blockage is caused by low hardness during operation, this method can be considered
2. Transforming into a large hole aerator (enterprises with low oxygen utilization rate, large fan margin, and good cost can consider) or a jet aerator (can only use the effluent from the monitoring pool to act as a power fluid, especially for high hardness wastewater, remember!)
At present, the author has encountered two situations:
1. Excessive mud pressure leads to an increase in ammonia nitrogen.
2. The sludge reflux is uneven, and the difference in sludge reflux between the two systems is too large, resulting in an increase in ammonia nitrogen on the side with less sludge reflux.
Analysis: Excessive sludge pressure and insufficient sludge reflux can both lead to a decrease in sludge age, as bacteria have a generation period. If SRT is lower than the generation period, the bacteria cannot aggregate in the system and form dominant bacterial species, resulting in the inability to remove corresponding metabolites. The general mud age is 3-4 times the bacterial generation period.
Solution:
1. Reduce water ingress or suffocation
2. Adding the same type of sludge (generally, using 1 and 2 pieces together is more effective)
3. If the problem is caused by imbalanced sludge reflux, reduce the inflow or suffocation of the problem series and ensure normal operation of the series, while returning some sludge to the problem series
6. Excessive ammonia nitrogen caused by ammonia nitrogen impact
This situation is generally encountered only in industrial wastewater or systems with industrial wastewater entering the domestic sewage pipeline network. The previous situation I encountered was that the temperature control of the upstream stripping tower decreased, causing a sudden increase in ammonia nitrogen in the incoming water, the denitrification system collapsed, the effluent ammonia nitrogen exceeded the standard, and the ammonia odor at the sewage treatment site was particularly strong (some free ammonia would escape during aeration).
Analysis: There is currently no clear explanation for ammonia nitrogen shock. The author analyzes that ammonia nitrogen shock is caused by excessive free ammonia (FA) in water. Although the effect of FA (free ammonia) on AOB (ammonia oxidizing bacteria/nitrite bacteria) is relatively weak, when the concentration of FA (free ammonia) is between 10-150mg/L, it begins to have an inhibitory effect on AOB (ammonia oxidizing bacteria/nitrite bacteria), And free ammonia (FA) is more sensitive to the impact of NOB (nitrite oxidizing bacteria/nitrate bacteria). Free ammonia (FA) has an inhibitory effect on NOB (nitrite oxidizing bacteria/nitrate bacteria) at 0.1-60mg/L. It is well known that nitrification reaction is completed jointly by nitrite bacteria and nitrate bacteria, and inhibition of nitrite bacteria can directly lead to the breakdown of the nitrification system.
Solution:
Under the condition of ensuring pH, the following three methods can achieve better and faster results simultaneously
1. Reduce the concentration of ammonia nitrogen in the system
2. Adding sludge of the same type
3. Stuffy explosion
7. Excessive ammonia nitrogen caused by low temperature
This situation often occurs in sewage treatment plants without insulation or heating in the north, because the water temperature is lower than the suitable temperature for nitrifying bacteria, and MLSS does not increase for slow metabolism in winter, resulting in a decrease in ammonia nitrogen removal rate.
Analysis: Bacteria have lower temperature requirements than humans, but there is also a bottom line, especially for autotrophic nitrifying bacteria. This situation is relatively rare in industrial wastewater because the temperature of wastewater produced by industrial production does not fluctuate greatly due to changes in environmental temperature. However, the temperature of domestic wastewater is basically controlled by environmental temperature. In winter, the inlet water temperature is very low, especially with a large temperature difference between day and night, which is often lower than the temperature required for bacterial metabolism, Causing bacterial dormancy and abnormal nitrification system.
Solution:
1. During the design phase, the pool body should be made underground (small sewage treatment is more suitable)
2. Advance increase of sludge concentration
3. If there is a homogeneous regulating tank for water inlet heating, it can be heated inside the tank to minimize fluctuations. If the water is directly fed, electric heating, steam heat exchange, or mixing can be used to increase the water temperature. This requires precise temperature control to control the fluctuations in the inlet temperature.
4. Aeration heating is relatively niche and has not been encountered yet. In fact, the temperature has already increased during air compression and blowing. If the aeration pipe can withstand it, it can be considered to heat compressed air to increase the temperature of the biochemical tank.
8. Process selection issues
I have encountered many friends who have consulted about ammonia nitrogen issues, but the root cause is often a problem with process selection. The processes used for denitrification are simple aeration tanks, contact oxidation, SBR, and so on. In fact, while ensuring that HRT (hydraulic retention time) and SRT (sludge age) are long enough, these processes can remove ammonia nitrogen. However, in practice, they are not economical and cannot be achieved!
Solution:
1. Extend HRT and SRT, such as transforming into MBR to increase mud age, etc
2. Add a denitrification tank in the front
2、 Why does total nitrogen exceed the standard!
1. Excessive ammonia nitrogen
Why does the ammonia nitrogen in the previous unit exceed the standard?
2. Lack of carbon source
In the process of nitrification and denitrification, the theoretical CN ratio required for removing TN is 2.86, but in actual operation, the CN (COD: TN) ratio is generally controlled at 4-6, lacking a carbon source, which is one of the most common reasons why I have encountered many friends who do not meet TN standards!
Solution: Add carbon source according to CN ratio of 4-6
3. Internal reflux r is too small
The full name of AO process is inverted nitrification denitrification process, and the denitrification efficiency of AO process is directly proportional to the internal reflux ratio! According to the denitrification efficiency formula, the higher the internal reflux ratio r, the higher the denitrification efficiency. Some sewage treatment internal reflux pumps are damaged or selected too small, which can lead to low denitrification efficiency!
Solution: Increase the internal reflux ratio r to 200-400%
4. Denitrification tank environment
The sign of this situation is that the DO in the denitrification tank is greater than 0.5, which destroys the anaerobic environment and allows facultative heterotrophic bacteria to preferentially utilize oxygen for metabolism. Nitrate nitrogen cannot be removed, leading to an overall increase in TN. The anaerobic environment in the denitrification tank is also damaged, which often leads to excessive ammonia nitrogen. The reason is that nitrifying bacteria cannot form dominant bacterial species. However, if the aeration tank is large enough, there is still no problem
Solution:
1. If the internal reflux flow is too large, resulting in excessive carrying of DO, reduce the internal reflux ratio or turn off the aeration at the internal reflux point.
2. The high DO caused by other issues, such as the high distance between the inlet and the water surface, which leads to falling and oxygenation, requires reducing the height difference, etc.
Some nitrogen-containing organic compounds cannot be removed by ordinary biochemistry, which is relatively rare, mainly on a certain type of wastewater. In this case, the main issue is process selection, without considering the process of organic nitrogen ammonification (conversion of organic nitrogen to ammonia nitrogen).
Solution:
1. Increase pre-treatment for hydrolysis and acidification
2. For those that cannot be broken by hydrolysis and acidification, advanced oxidation pretreatment should be added
Related Products
Biological nitrogen removal process of low temperature wastewater
2024-05-28
Prevention and treatment of calcium carbonate scaling in reverse osmosis operation
2024-05-22
Treatment of pyrazolone production wastewater - bipolar membrane electrodialysis process
2024-05-20
How much salt does sewage contain that can enter the biochemical system?
2024-05-17
Huanke Environmental Protection Technology
HOTLINE:
Address:Gongye 1st Street, Weicheng District, Weifang City, Shandong Province China
Contact:Zhang Gong
Phone:+86-18865361829
Email:sdhuanke@163.com


Consult
Copyright © 2023 Shandong Huanke Environmental Protection Technology Co., Ltd