22
2023
-
11
Introduction to Wet Oxidation Technology
Author:
Huanke
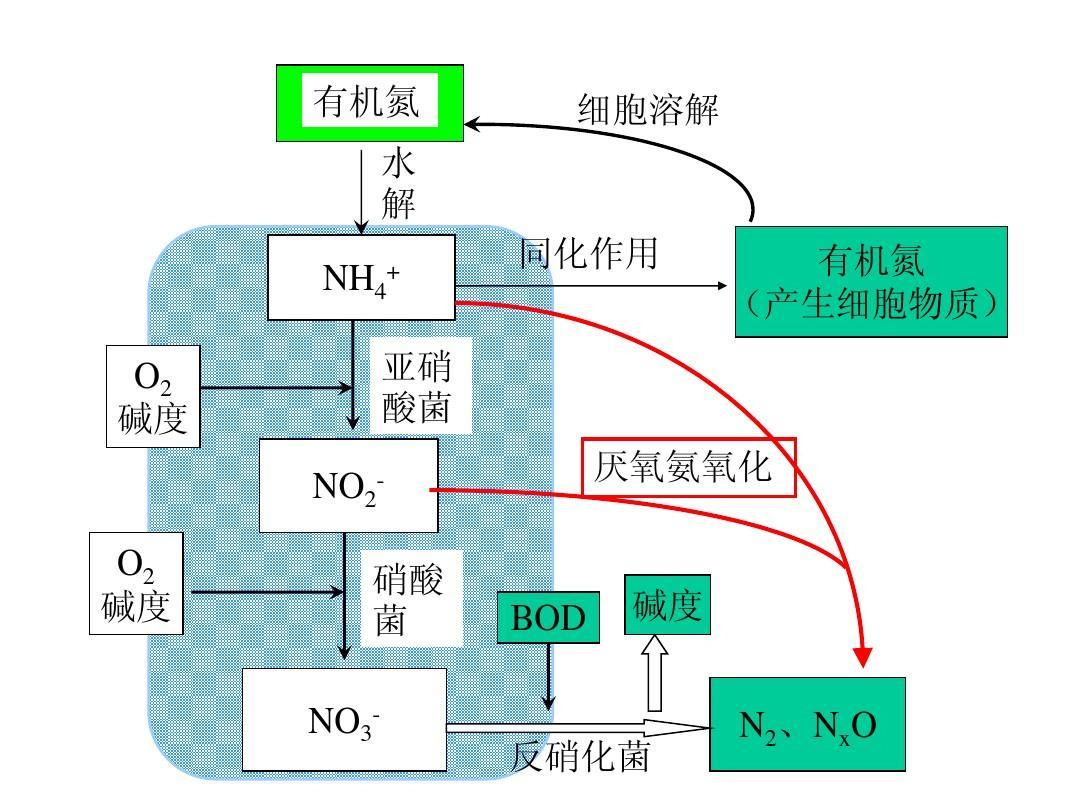
Wet oxidation technology has become an effective water treatment method for treating toxic, harmful, and high concentration organic wastewater since the 1950s. However, in-depth research on this technology began in China in the 1980s. This technology requires the chemical process of converting organic pollutants into inorganic or organic small molecules such as carbon dioxide and water in the liquid phase under high temperature and pressure conditions, using oxygen in the air as an oxidant.
The characteristic of wet oxidation technology is that it has a wide range of applications and can effectively treat various types of high concentration organic wastewater without selection. Under appropriate temperature and pressure conditions, the COD treatment rate can reach over 90%; At the same time, it has a fast oxidation rate for organic pollutants, usually only taking 30 to 60 minutes. In addition, wet oxidation technology has the characteristic of less secondary pollution.
Wet oxidation technology is suitable for treating industrial wastewater with high concentration and low flow rate, but it is not economical for domestic wastewater with low concentration and high flow rate. In recent years, people have made improvements to traditional wet oxidation technologies, such as using efficient and stable catalysts for wet catalytic oxidation, adding strong oxidants such as hydrogen peroxide and ozone, and utilizing the excellent characteristics of supercritical water to accelerate the reaction process. These improvements have greatly improved the working conditions and degradation efficiency of wet oxidation, making it more practical and economical.
In the treatment of wastewater containing organic phosphorus and organic sulfur pesticides, wet oxidation technology converts organic sulfur into sulfuric acid and organic phosphorus into phosphoric acid under conditions of 180 to 230 ℃ and 7 to 15 MPa. When the reaction temperature is 204 to 316 ℃, the decomposition rates of various compounds, including hydrocarbons and oxides, are close to 99%. For difficult to oxidize chlorides such as polychlorinated biphenyls, DDT, and pentachlorophenol, the removal rate can reach over 85% using a mixed catalyst for wet air oxidation technology. In the treatment of papermaking black liquor, controlling the reaction temperature to 150 to 350 ℃ and pressure to 5 to 20 MPa, the COD removal rate of the treated wastewater can reach over 90%.
The wet oxidation reaction is carried out under the conditions of high-pressure air injection and reaction temperature of 300 ℃. It is generally divided into three stages: thermal decomposition stage, local oxidation stage, and complete oxidation stage. In the thermal decomposition stage, high molecular weight organic matter dissolves and hydrolyzes (but is not oxidized), and the reaction rate increases with increasing temperature. During local oxidation, high molecular weight organic compounds oxidize and decompose into intermediate products with lower molecular weight. Such as formic acid, acetic acid, formaldehyde, etc. In the final stage of complete oxidation, the intermediate organic products are further oxidized into carbon dioxide and water. The oxidation stage is mainly determined by the activation energy of organic matter oxidation and the conditions such as temperature and pressure during treatment. The heat generated during the reaction can be used to maintain the high temperature of the reaction. Under the action of high temperature and pressure, the reaction rate is accelerated, gas-liquid two-phase mass transfer is strengthened, and the solubility of oxygen in water is also increased.
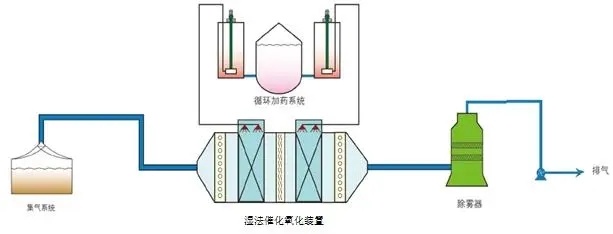
The common industrial scale wet oxidation process is as follows: the treated wastewater is pressurized by a high-pressure pump and then heated to the required temperature in a heat exchanger before entering the reactor; At the same time, air or pure oxygen is compressed into the reactor through an air compressor. In the reactor, the oxidizable pollutants in the wastewater are oxidized by oxygen. After the reaction products are discharged from the reactor, they first enter the heat exchanger and are cooled while heating the raw water; Then, the reaction products enter the gas-liquid separator, and the gas phase (mainly nitrogen, carbon dioxide, and a small amount of unreacted low molecular organic matter) and liquid phase are separated and discharged separately.
The significant characteristics of wet oxidation process are wide range of organic matter treatment, good effect, short reaction time, small reactor volume, almost no secondary pollution, and the ability to recover useful substances and energy. However, the main limiting factors for the development of wet oxidation are high equipment requirements and large one-time investment.
Related Products
Biological nitrogen removal process of low temperature wastewater
2024-05-28
Prevention and treatment of calcium carbonate scaling in reverse osmosis operation
2024-05-22
Treatment of pyrazolone production wastewater - bipolar membrane electrodialysis process
2024-05-20
How much salt does sewage contain that can enter the biochemical system?
2024-05-17
Huanke Environmental Protection Technology
HOTLINE:
Address:Gongye 1st Street, Weicheng District, Weifang City, Shandong Province China
Contact:Zhang Gong
Phone:+86-18865361829
Email:sdhuanke@163.com


Consult
Copyright © 2023 Shandong Huanke Environmental Protection Technology Co., Ltd